- ACT & Applications
The paper describing the Alexandria Chemistry Toolkit was just published in Digital Discovery. This is a great for future applications of force field science using the ACT. In fact, a first paper with an ACT application was published already a few weeks ago in Phys. Chem. Chem. Phys., as a communication. Using the ACT, we investigate the impact of charge distributions on electrostatic interactions and propose to combine a positive point charge with a negative Gaussian distributed charge.
- Application of ACT to Electrostatics
Method developers do not usually discover things, but in this paper we describe our finding that the popular method for deriving partial charges from fitting to the electrostatic potential is fundamentally flawed due to lack of information. We then proceed to apply the ACT to derive alternative models for predicting electrostatic and induction interactions.
- Alexandria Chemistry Toolkit
Now we have started the publication process for the ACT. First out is the User & Reference manual that you can download from Zenodo.
The ACT software has been released as free and open source at github.
A manuscript about the ACT has been submitted and a preprint can be found at ChemRxiv and supporting information at Zenodo.
- VR Grant
It is great to be able to announce that the project “Machine Learning Physics-based Force Fields” by David van der Spoel was granted 4.4MSEK by the Swedish Research Council. Research on Force Field Science will continue with a new graduate student or post-doc.
- New force field papers
Two new fundamental papers were published recently. First, one about noble gases where we were able to fit very accurate quantum chemistry data, and in combination with a three-body potential, could reproduce melting points for crystals. Since the interactions are weak this is quite a feat! The paper also investigates combination rules and analytical potentials for Van der Waals interactions.
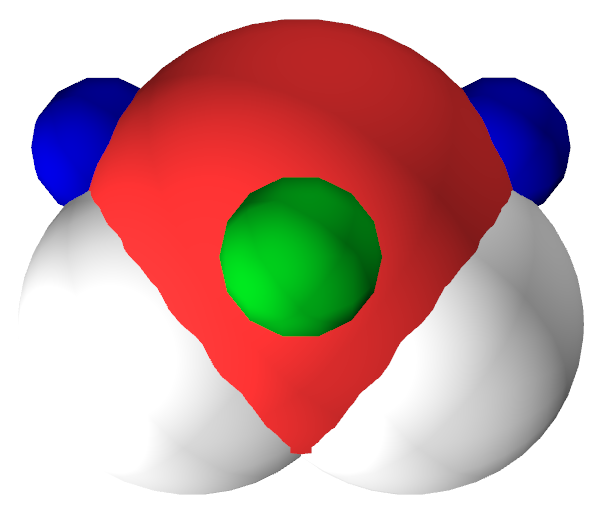
Second, we studied the anisotropy in exchange interactions by probing hydrogen halides and water with a Helium atom. This showed that there is considerable anisotropy due to sigma-holes, both on halides and on water. The image below shows the lone-pairs in green and the sigma-holes in blue.